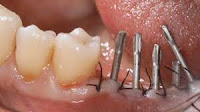
Which material has best biocompatibility Intraorally:
a- Cobalt chromium.
b- Titanium***
c- Nickle chromium.
d- Gold... Palladium.
Chemical element, Ti, of atomic number 22 and atomic weight 47.90.
Chemical element, Ti, of atomic number 22 and atomic weight 47.90.
While its chemical behavior shows many similarities with that of silicon and zirconium, as an element of the first transitional group, the chemistry of the aqueous solution, especially of the lower oxidation states, bears some resemblance to that of chromium and vanadium.
The main state of valence is 4+, although states 3+ and 2+ are also known, which are less stable.
The main state of valence is 4+, although states 3+ and 2+ are also known, which are less stable.
The element burns in the air when heated to obtain dioxide, TiO2, and when combined with halogens.
It reduces water vapor to form dioxide and hydrogen, and reacts similarly with hot concentrated acids, although it forms the trichloride with hydrochloric acid.
The metal absorbs hydrogen to give approximately TiH2 compositions, and forms the nitride, TiN, and the carbide, TiC. The sulfide TiS2 is known, as well as the lower oxides, Ti2O3 and TiO, and the sulfides Ti2S3 and TiS. Salts of the three valence states are known.
Titanium dioxide, TiO2, is commonly found in a black or tan shape known as rutile.
The natural forms found less in nature are the anatasite and the broochite. Both pure rutile and anatasite are white.
The basic black oxide, FeTiO3, is found naturally as the mineral called ilmenite; This is the main commercial source of titanium.
Titanium dioxide is widely used as a white pigment in exterior paints because it is chemically inert, due to its great coating power, its opacity to UV light damage and its self-cleaning capacity.
The dioxide has also been used as a whitening and opaque agent in porcelain enamels, giving a final finish of great brightness, hardness and acid resistance.
The alkaline earth titanates have some remarkable properties.
The alkaline earth titanates have some remarkable properties.
The level of dielectric constants ranges from 13 for MgTiO3, and several thousand for solid solutions of SrTiO3 in BaTiO3.
The barium titanate also has a dielectric constant of 10,000 near 120ºC (250ºF), which is its Curie point; it has low dielectric hysteresis.
The ceramic transducers containing barium titanate compare favorably with Rochelle salt and quartz, with respect to the thermal stability in the first case, and the strength of the effect and the ability to form the ceramic in various forms in the second case.
The compound has been used as a generator of ultrasonic vibrations and as a sound detector.